Relative Atomic Mass of Magnesium
Magnesium-sulfur batteries with high massvolumetric energy density great safety and low cost have been considered as one of most potential RMBs. Calculate the relative atomic mass atomic weight of chlorine.
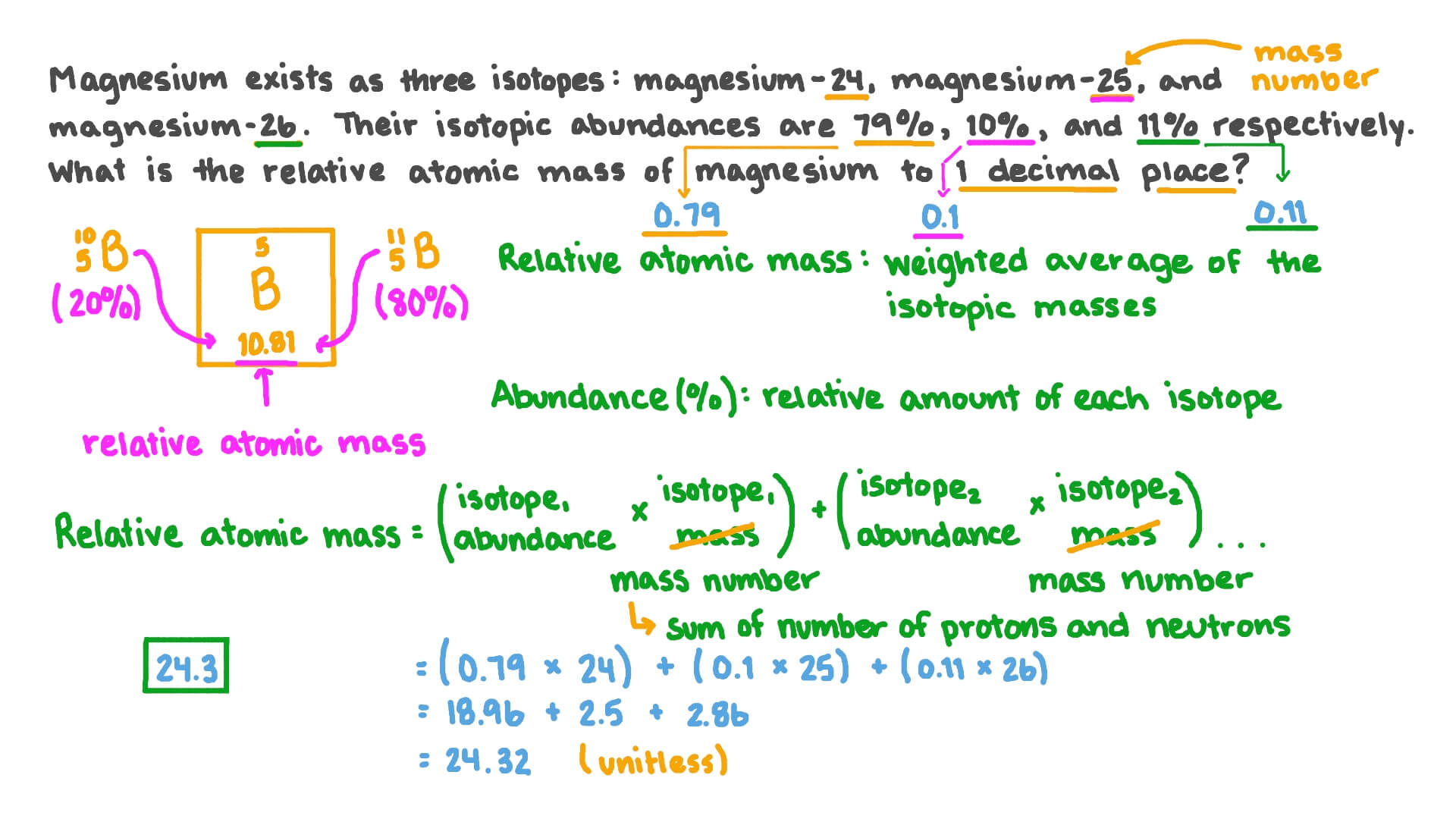
Question Video Calculating The Relative Atomic Mass Of Magnesium From Isotopic Abundances Nagwa
22 Evolution of Atomic Theory.

. For a complete list see abundance of elements in Earths crust. The graph at right illustrates the relative atomic-abundance of the. 24 12 Mg and 26 12 Mg are symbols of two isotopes of magnesium.
A r of Mg 24 A r of N 14 A r of O 16 Reveal answer. The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total mass of the atom. This is approximately the sum of the number of protons and neutrons in the nucleus.
143 Relative Strengths of Acids and Bases. 34 Other Units for Solution. Plate tectonics is the theory that Earths outer shell is divided into several plates that glide over Earths mantle.
They will also require the relative atomic masses. For example magnesium Mg exists as a mixture of three isotopes each of which has an atomic number of 12 and a mass number of 24 25 and 26 respectively. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element.
Build an atom out of protons neutrons and electrons and see how the element charge and mass change. The relative atomic mass of an element. Iron atomic mass is 55845 u.
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Calculate the relative formula mass M r of magnesium nitrate MgNO 3 2. 32 Determining Empirical and Molecular Formulas.
Other elements occur at less than 015. 3 Composition of Substances and Solutions. They should divide mass by the atomic mass for each element.
23 Atomic Structure and Symbolism. Know the physical and chemical properties density boiling and melting point along with the uses of Iron on BYJUS. By using this chemists work out the chemical formula.
Magnesium is 24 and oxygen is 16. Relative atomic mass The mass of an atom relative to that of carbon-12. Carbon is taken as the standard atom and has a relative atomic mass A r of 12.
174 convert the given mass of a substance to the amount of the substance in moles and vice versa by using the relative atomic or formula mass. 21 Early Ideas in Atomic Theory. Nowadays these issues including insulating nature of elemental sulfur sluggish redox kinetics between S and Mg 2 severe volume changes low conductivity and low utilization of polysulfides etc still extremely.
The Earth retains oxygen as the second-largest component of its mass and largest atomic. These values tell you that a magnesium atom has twice the mass of a carbon atom. Compare the atoms of these isotopes with respect to.
Where more than one isotope exists the value given is the abundance weighted average. 31 Formula Mass and the Mole Concept. The composition of their nuclei.
25 magnesium 24 potassium 20 and titanium 061. Density is the mass of a substance that would fill 1 cm 3 at room temperature. Atomic number Atomic mass and Relative atomic mass Isotopes and radioactive decay isotopes half-life.
Then play a game to test your ideas. Iron Fe - Iron is represented as Fe and has an atomic number of 26.
What Is The Atomic Mass Of Magnesium Quora
What Is The Relative Atomic Mass Of Magnesium Ions It Has Three Isotopes The Mass Numbers Are 24 25 26 And The Abundance Is 79 0 10 0 And 11 0 Quora

No comments for "Relative Atomic Mass of Magnesium"
Post a Comment